Renuvion | ThermiRF | ThermiVa | Cristal Cryolipolysis | Cristal Pro® | Regenera | Plexr | Renova

Renuvion

BENEATH THE SURFACE
REAL RESULTS
Abdominal
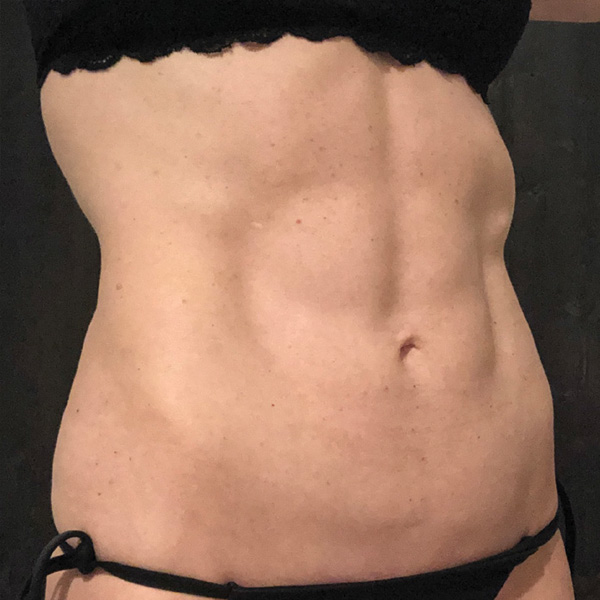
Abdominal Case Example #1
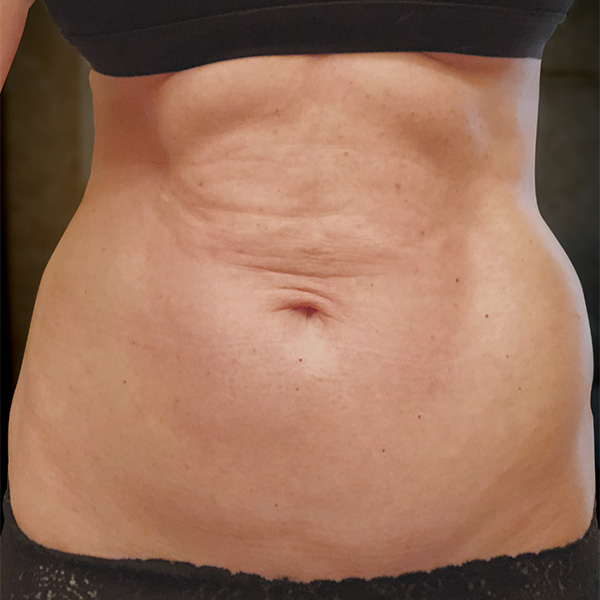
Before
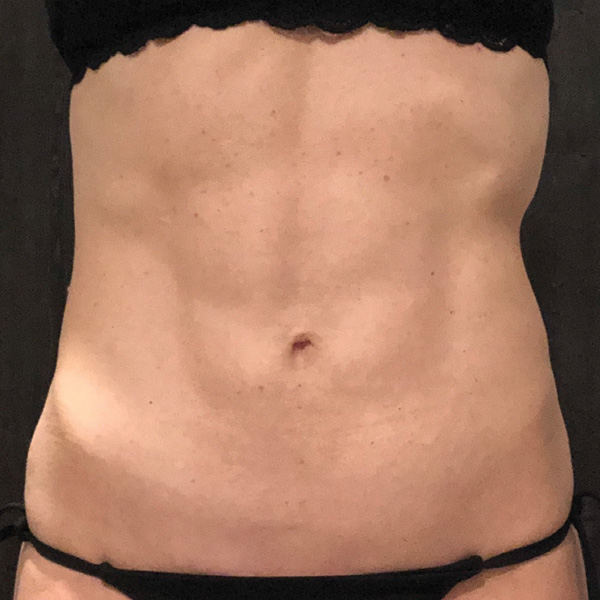
2 yrs Post-Op*
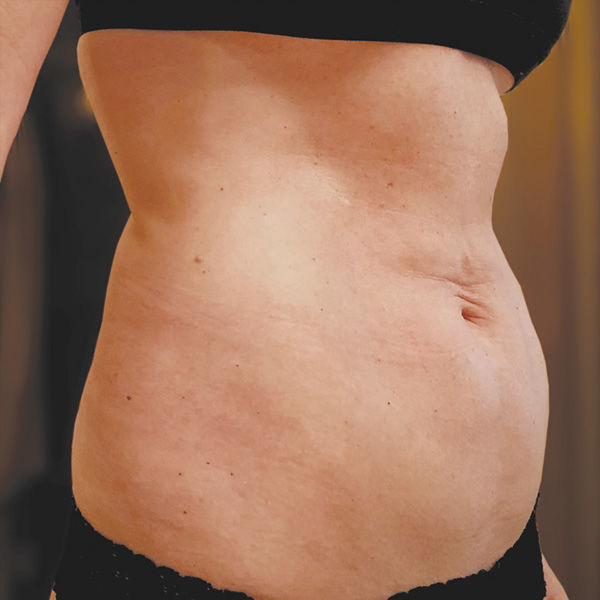
Before
2 yrs Post-Op*
Procedure Type: Ultrasonic liposuction
Amount of Fat Removed: 150cc
Renuvion used subdermally on entire abdomen
*No change in weight, diet or exercise
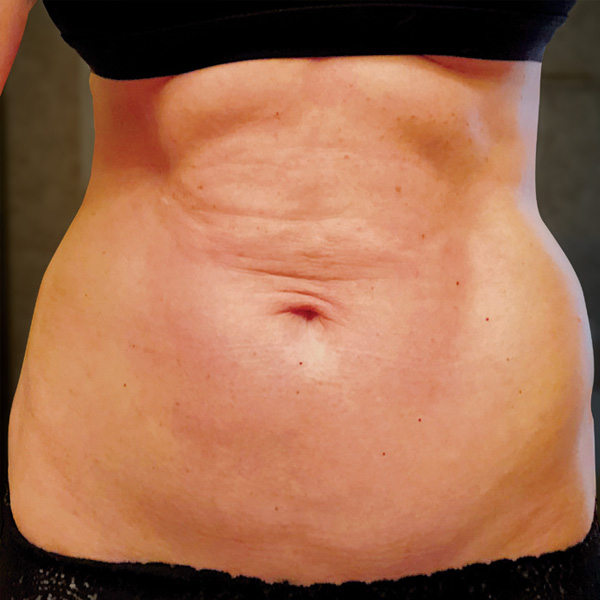
Before
1 yr 2 mo Post-Op*
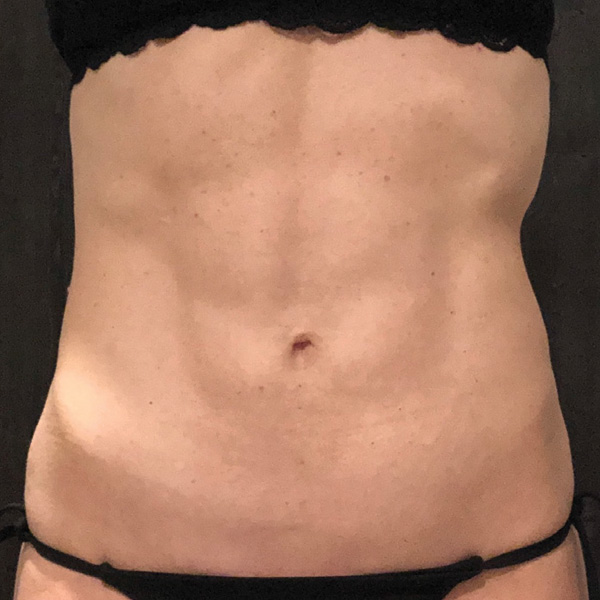
Before
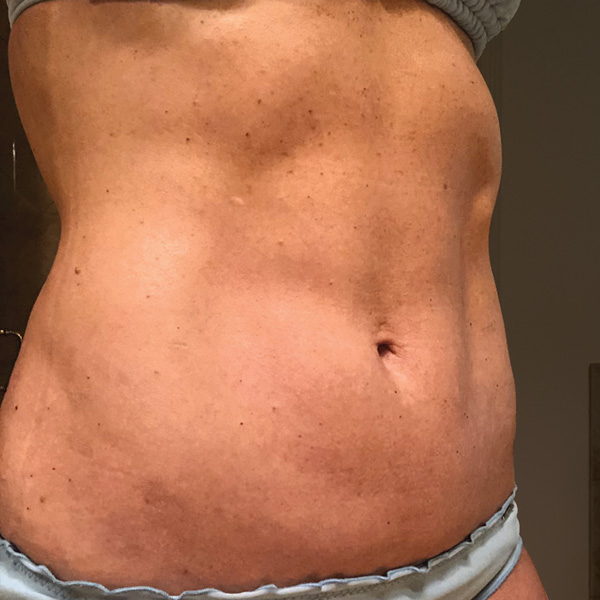
1 yr 2 mo Post-Op*
Procedure Type: Ultrasonic liposuction
Amount of Fat Removed: 150cc
Renuvion used subdermally on entire abdomen
*No change in weight, diet or exercise
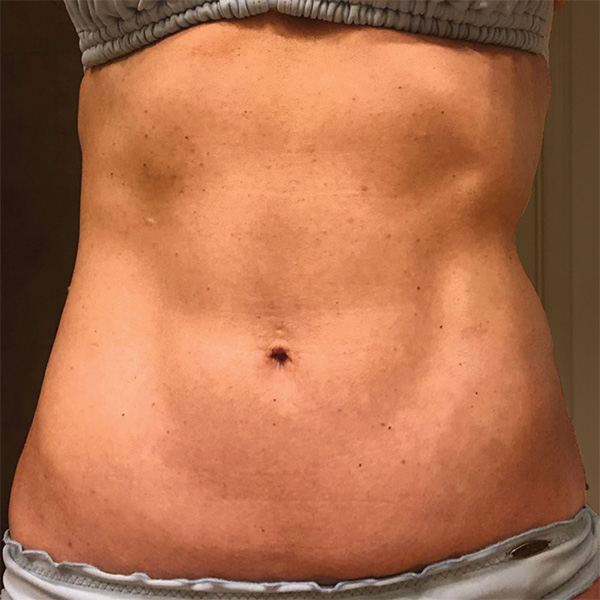
Abdominal Case Example #3
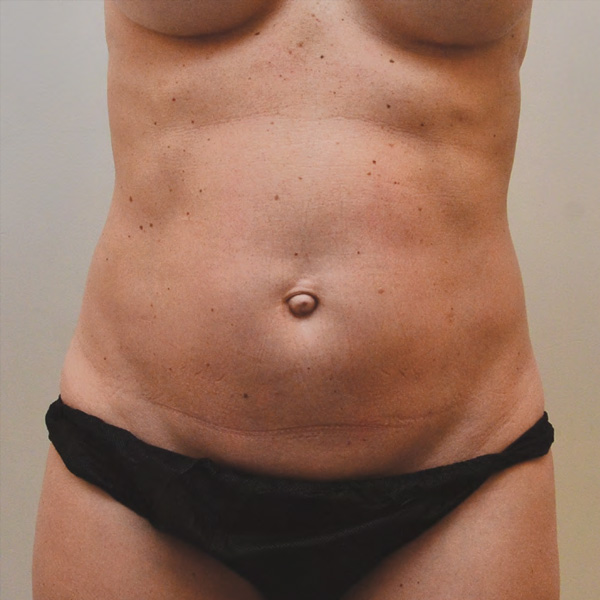
Before
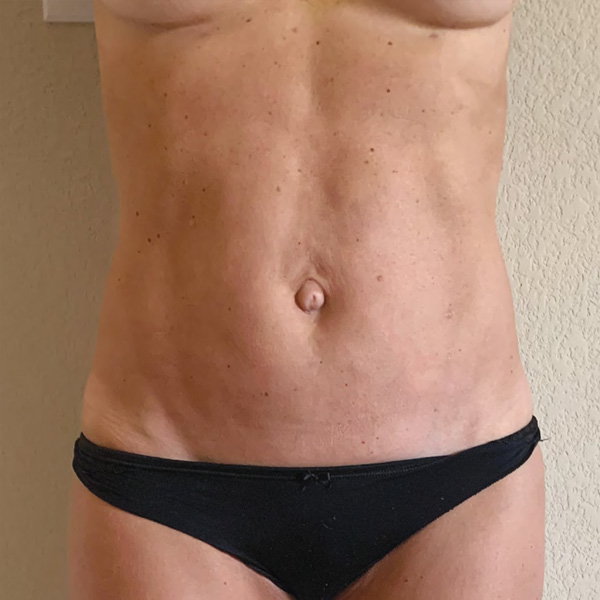
4 Weeks Post-Op
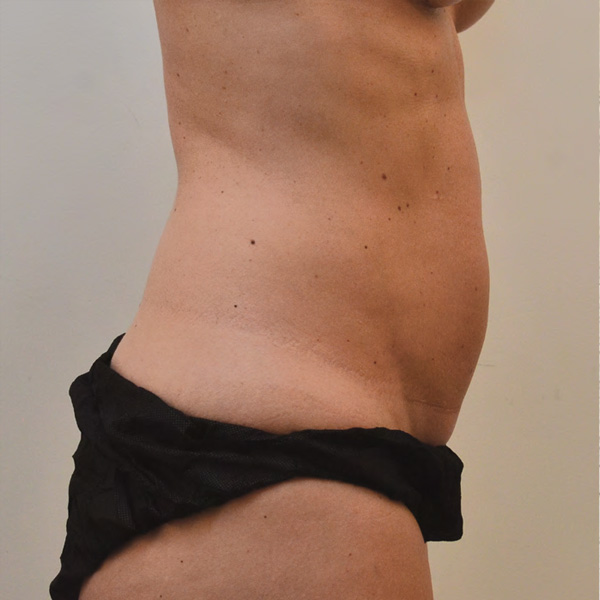
Before
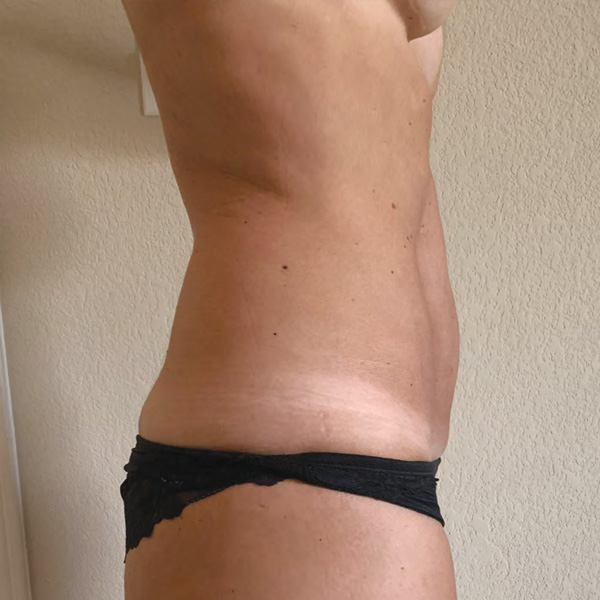
4 Weeks Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 150cc
Renuvion used subdermally on entire abdomen
Abdominal Case Example #4
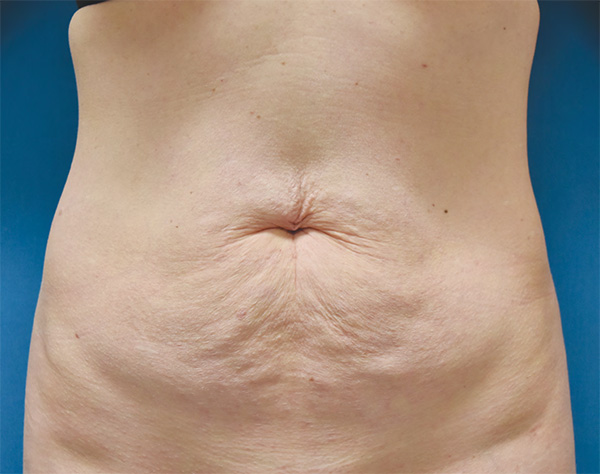
Before
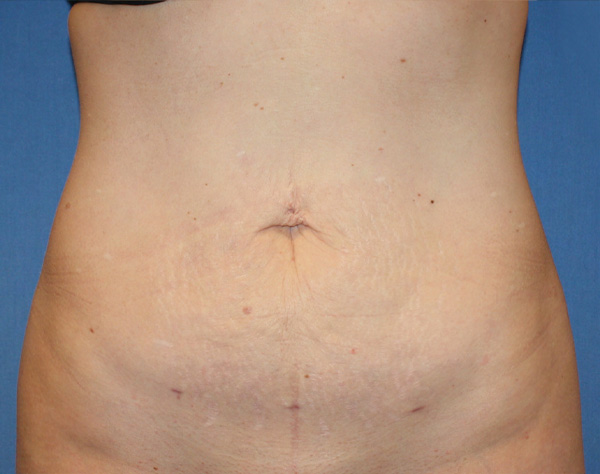
10 Weeks Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 175cc
Renuvion used subdermally on entire abdomen
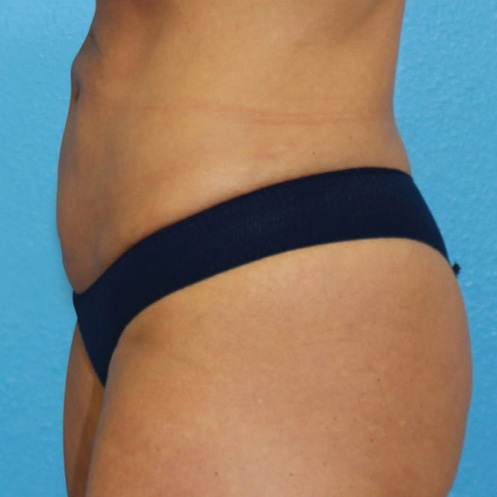
Abdominal Case Example #8
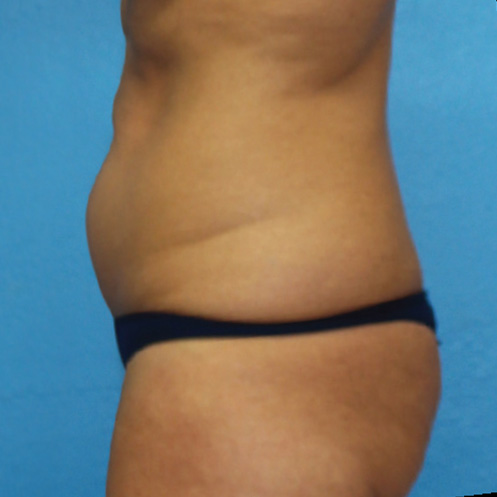
Prior to Any Procedure
6 Months Post Bipolar RF + Liposuction
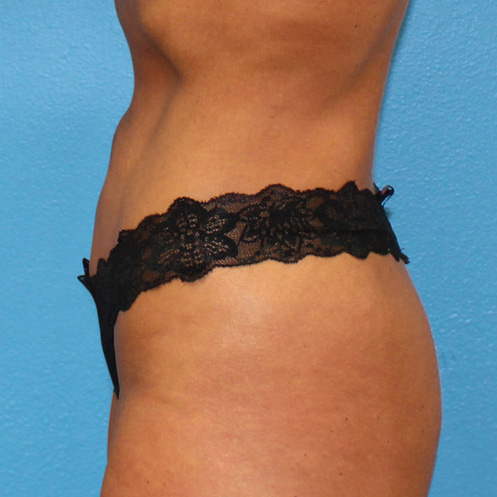
6 Months Post* Bipolar RF + Liposuction
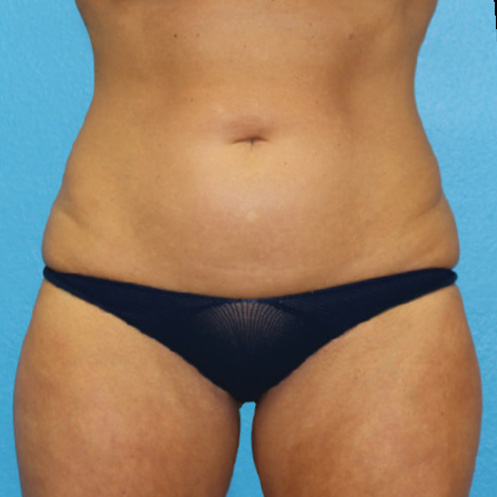
Prior to Any Procedure
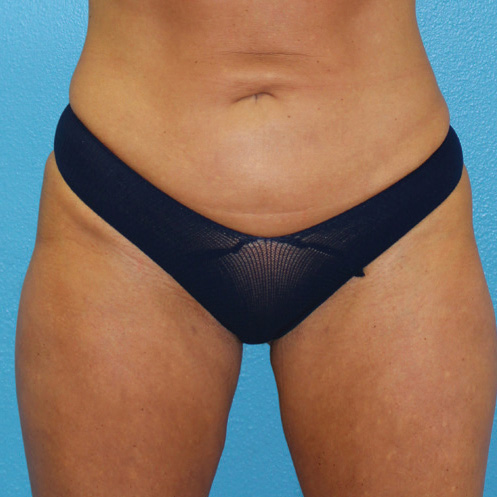
6 Months Post Bipolar RF + Liposuction
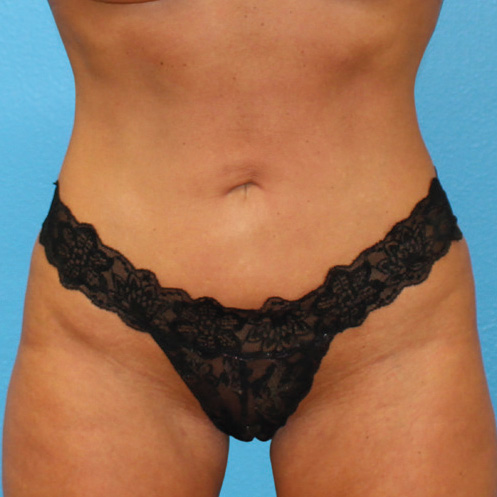
6 Months Post* Bipolar RF + Liposuction
Procedure Type: Power-assisted liposuction
Amount of Aspirate Removed:
• During Bipolar RF Procedure: 600cc
• During Renuvion Procedure: 50cc
Renuvion used subdermally on entire abdomen
Abdomen Case Example #9
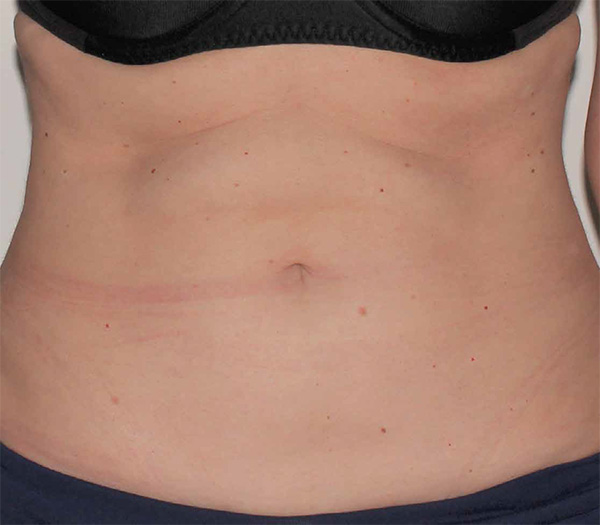
Before
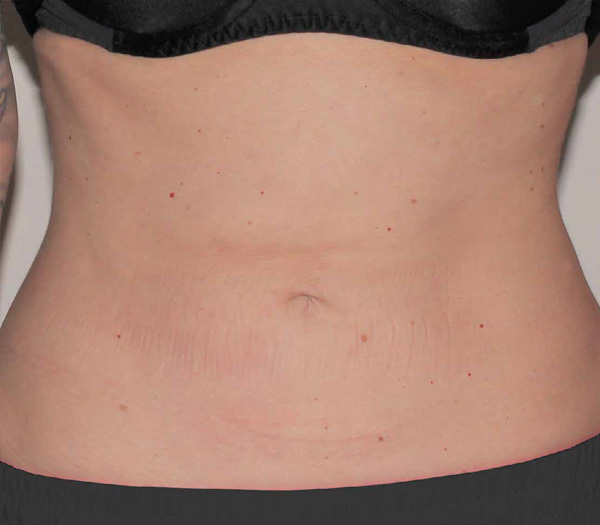
1 Year Post-Op
Procedure Type: Suction-assisted liposuction
Amount of Fat Removed: 1100cc
Renuvion used subdermally on abdomen
Arms
Arms Case Example #1

Before

6 Weeks Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 600cc
Renuvion used subdermally on anterior and posterior arms
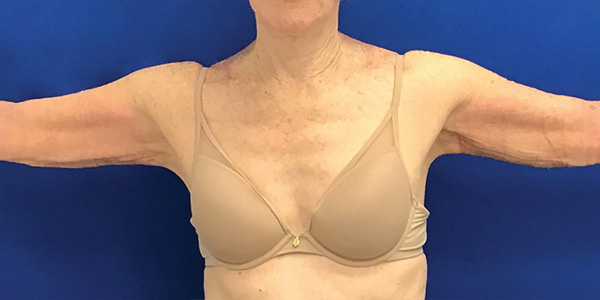
Arms Case Example #2
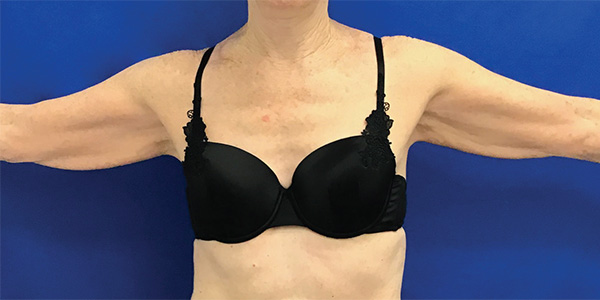
Before
6 Weeks Post-Op
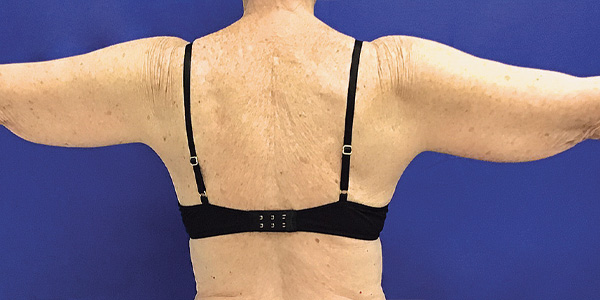
Before
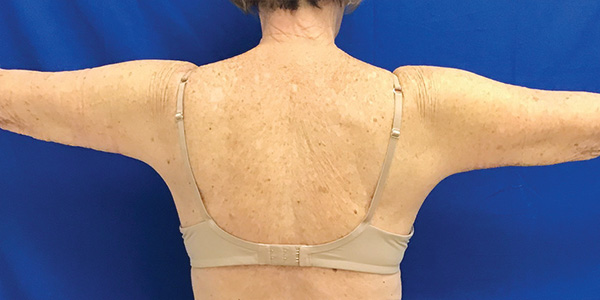
6 Weeks Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 150cc
Renuvion used subdermally on arms
Arms Case Example #3
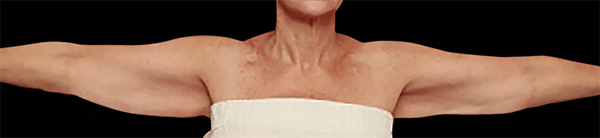
| Before | 1 yr 3 mo Post-Op |
| Liposuction only | Renuvion + Liposuction |
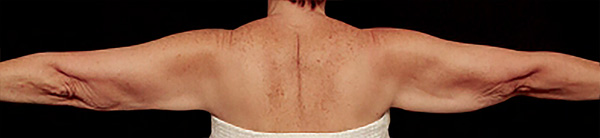
| 1 yr 3 mo Post-Op | Before |
| Renuvion + Liposuction | Liposuction only |
Procedure Type: Power-Assisted Liposuction
Amount of Fat Removed: 250cc
Renuvion used subdermally on left arm
Back
Back and Flanks Case Example #1
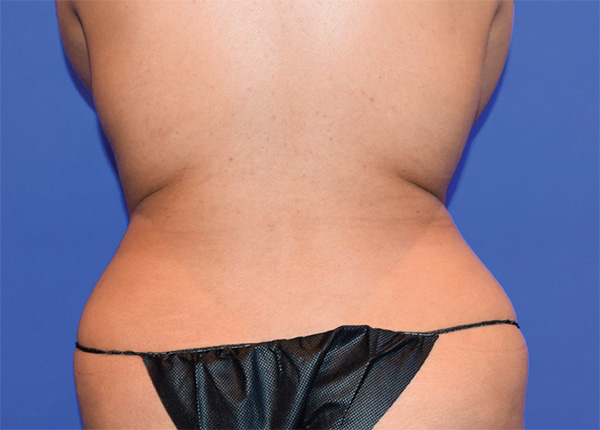
Before
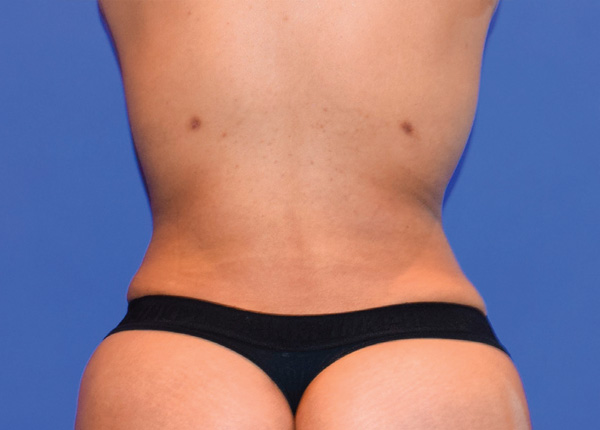
4 Weeks Post-Op*
Procedure Type: Power Assisted Liposuction
Amount of Fat Removed: 1200cc
Renuvion used subdermally on back and flanks
*Patient also had fat transfer to buttocks
Back Case Example #3
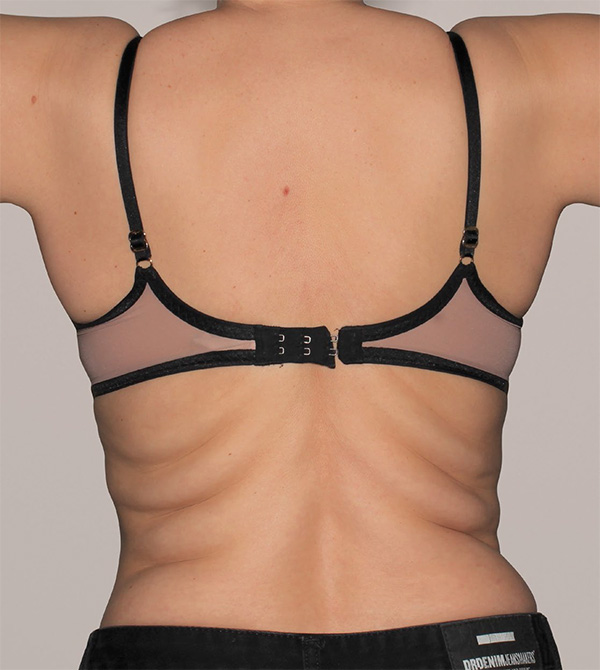
Before
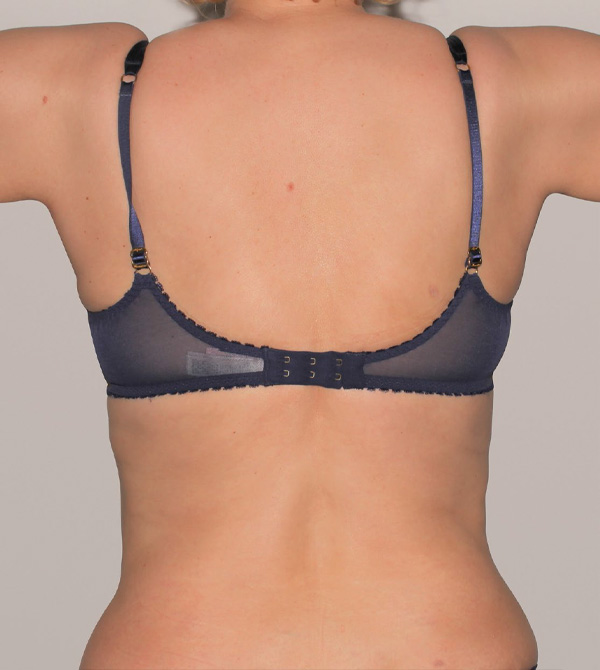
6 Months Post-Op
Procedure Type: Suction-assisted liposuction
Amount of Fat Removed: 500cc
Renuvion used subdermally on back
Back Case Example #5
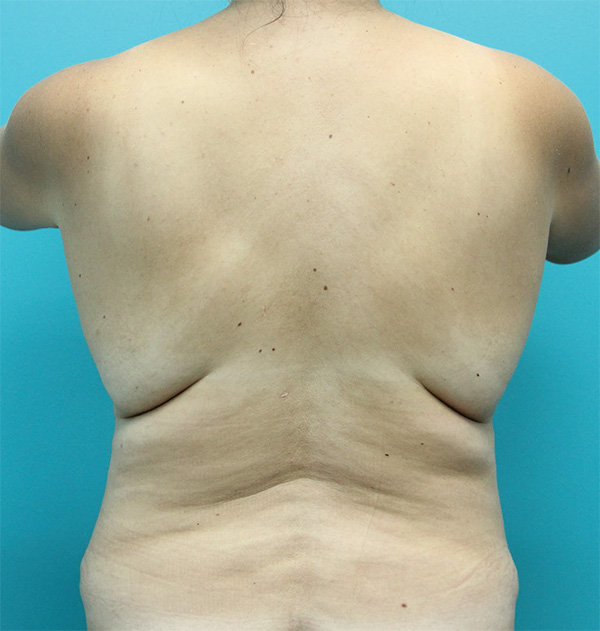
Before
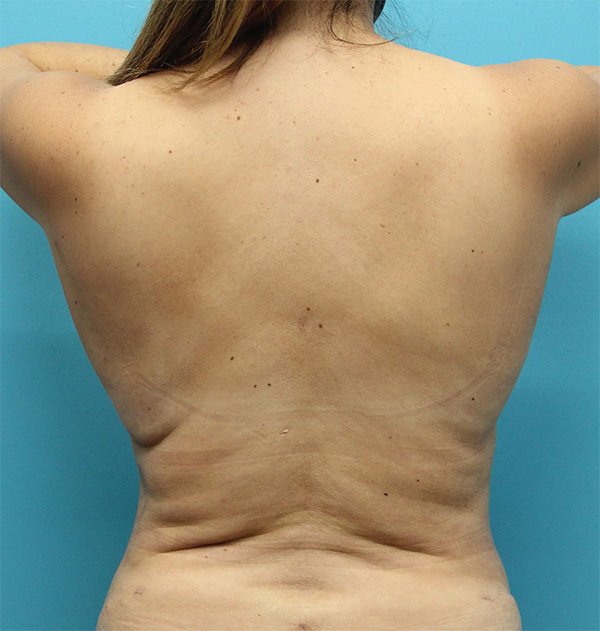
6 Months Post-Op
Procedure Type: Suction-assisted liposuction
Amount of Fat Removed: 300cc
Renuvion used subdermally on mid and upper back
Body
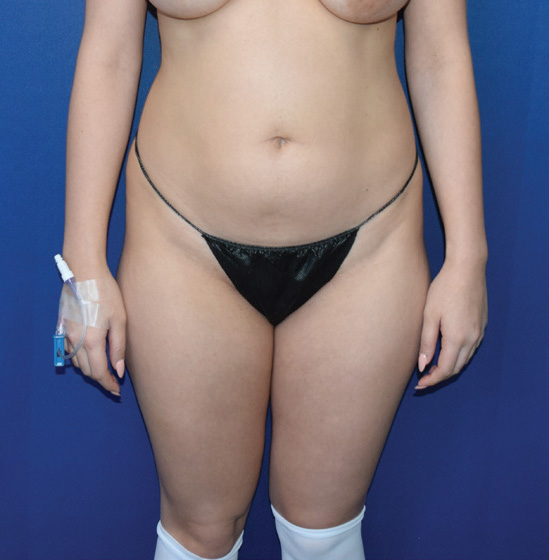
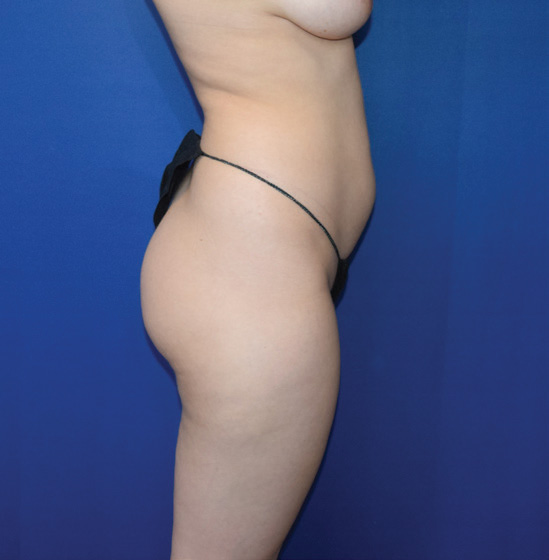
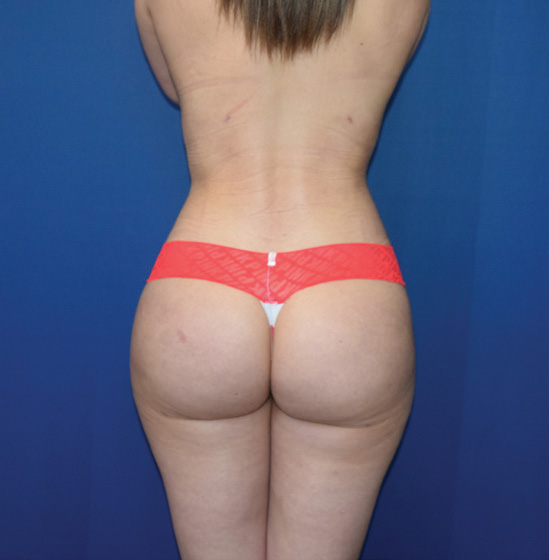
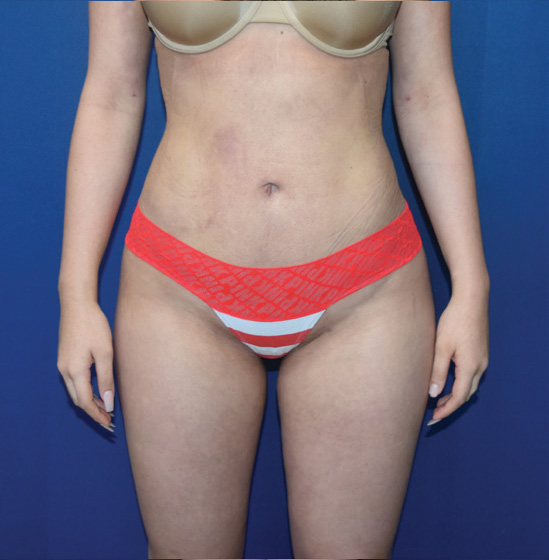
Body Case Example #1

Before
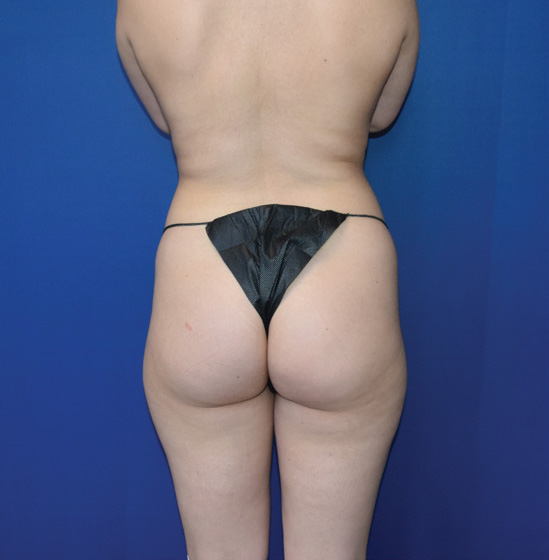
Before
Before
Before

6 Weeks Post-Op*
6 Weeks Post-Op*
6 Weeks Post-Op*
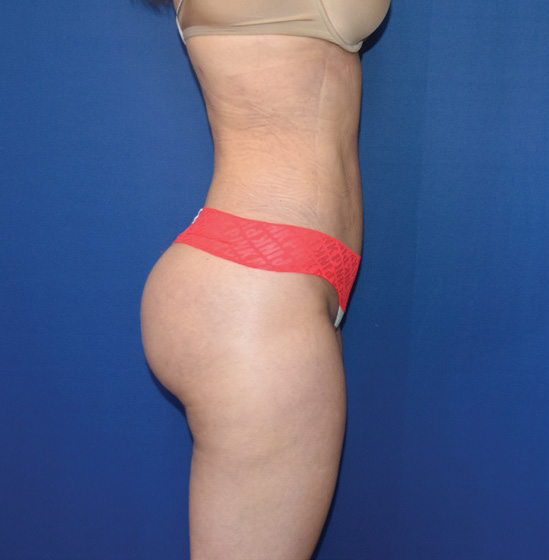
6 Weeks Post-Op*
Procedure Type: Power-assisted liposuction
Amount of Fat Removed: 1800cc
Renuvion used subdermally on abdomen, back and inner thighs
*Patient also had fat transfer to buttocks
Neck
Neck Case Example #1
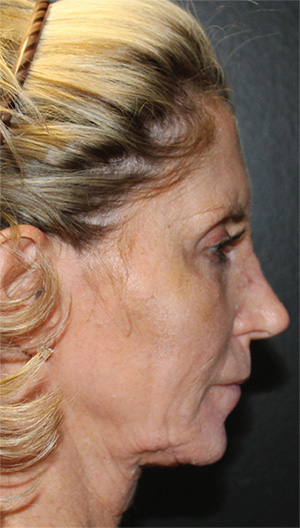
Before
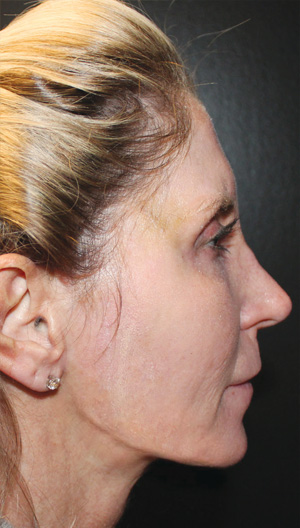
2 Weeks Post-Op
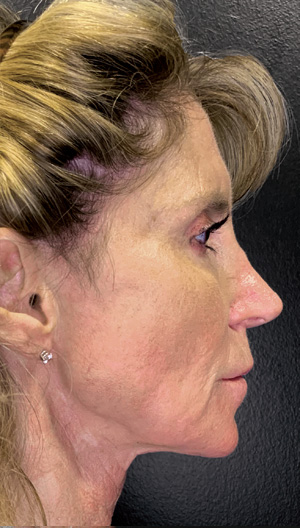
1 yr 6 mo Post-Op
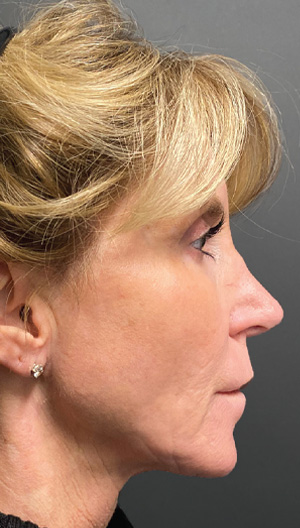
2 yrs Post-Op
Procedure Type: Ultrasonic liposuction
Renuvion used subdermally on jowls and neck
Neck Case Example #3
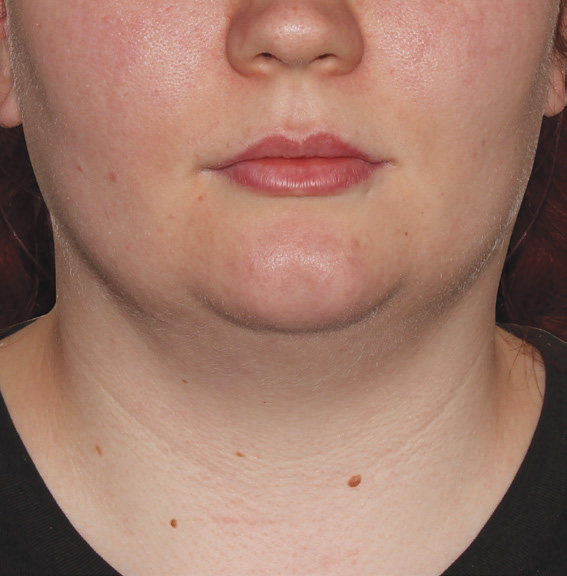
Before
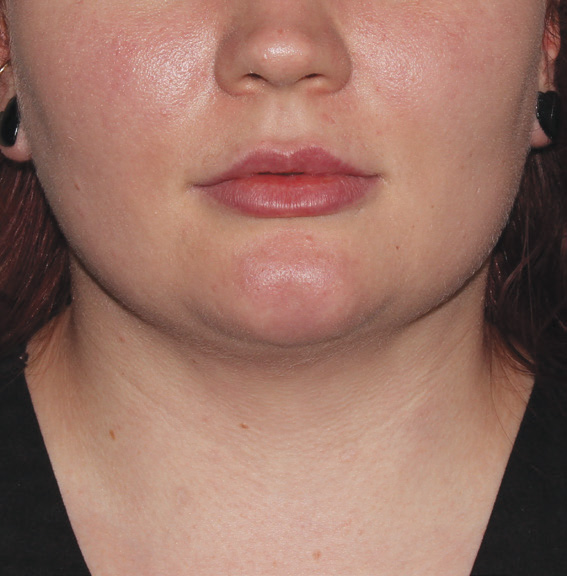
6 Weeks Post-Op
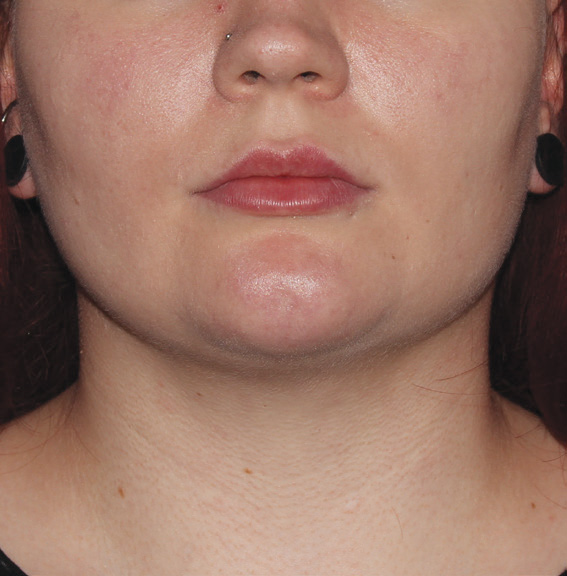
12 Weeks Post-Op
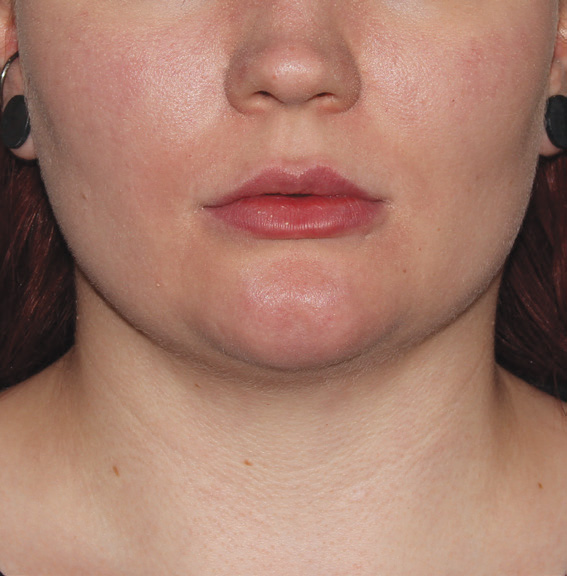
24 Weeks Post-Op

Before
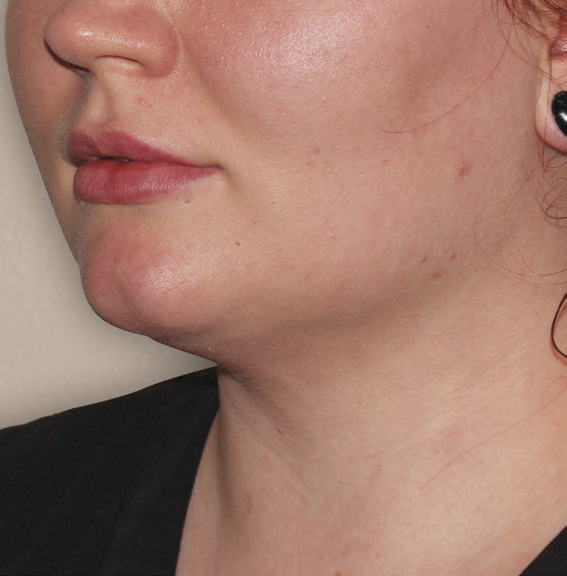
6 Weeks Post-Op
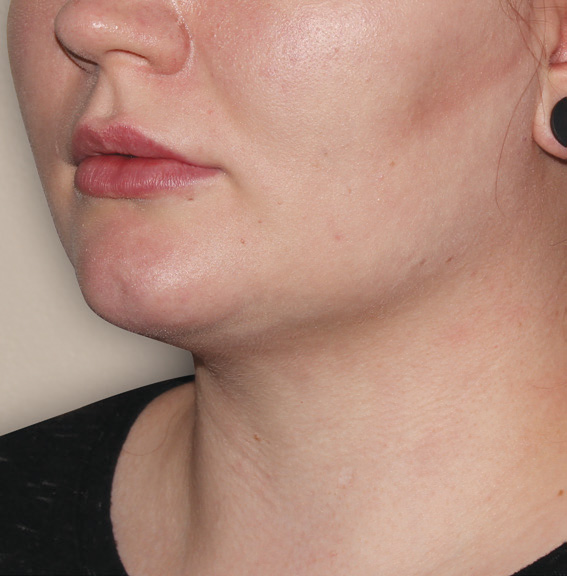
12 Weeks Post-Op
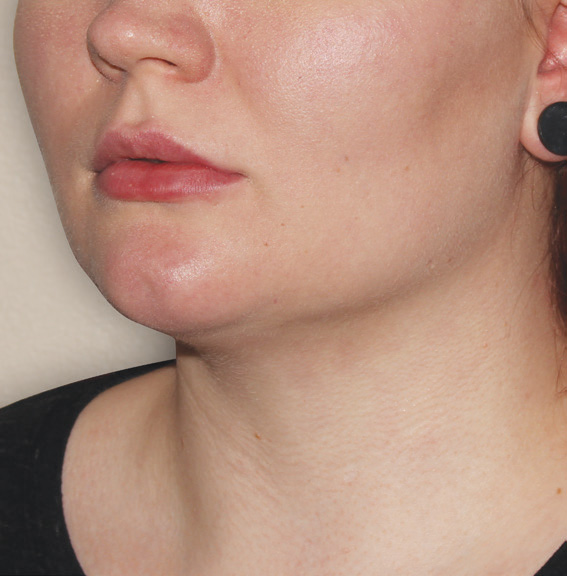
24 Weeks Post-Op
Procedure Type: Suction Assisted Lipectomy
Amount of Fat Removed: 60-70cc
Renuvion used subdermally on neck and jowls
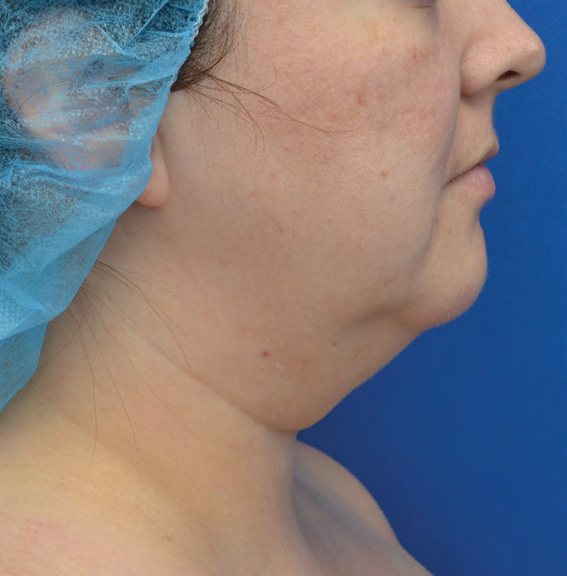
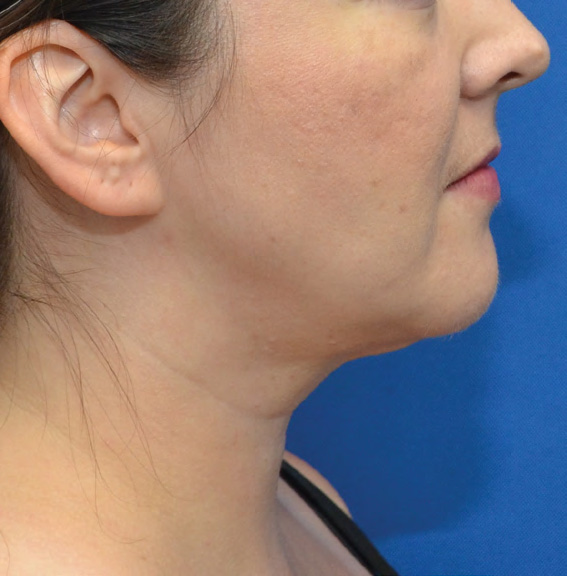
Neck Case Example #6
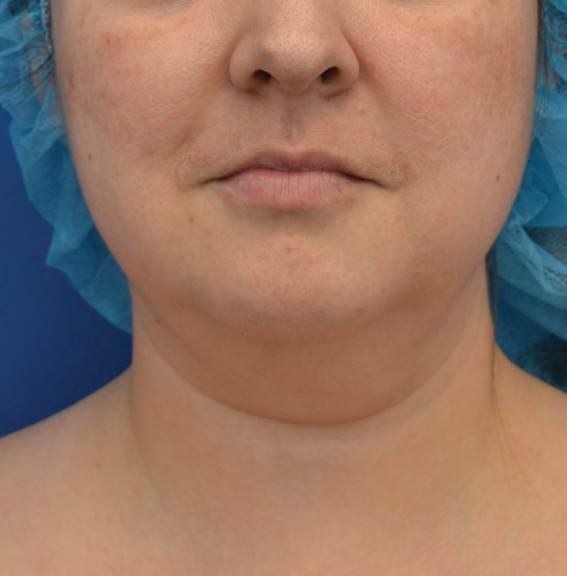
Before
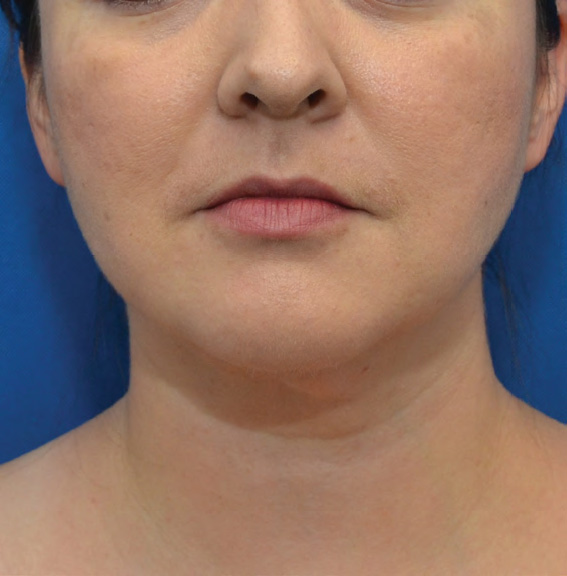
3½ Months Post-Op
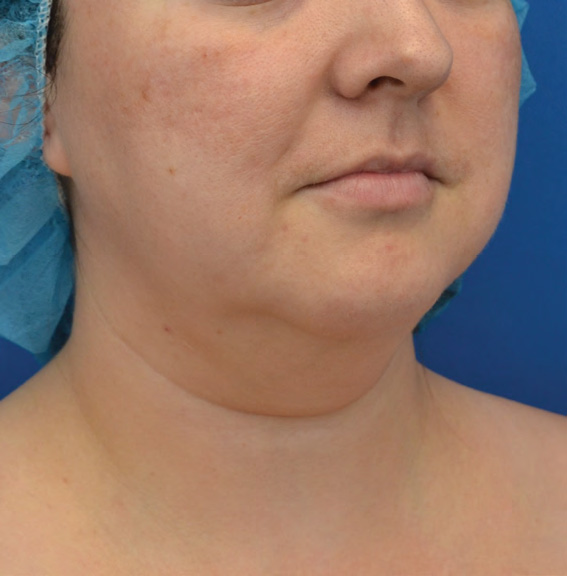
Before
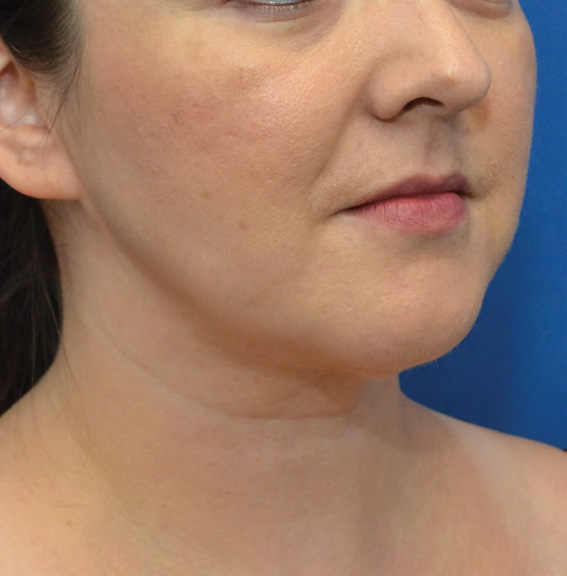
3½ Months Post-Op
Before
3½ Months Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 100cc
Renuvion used subdermally on neck
Thigh
Thigh Case Example #1
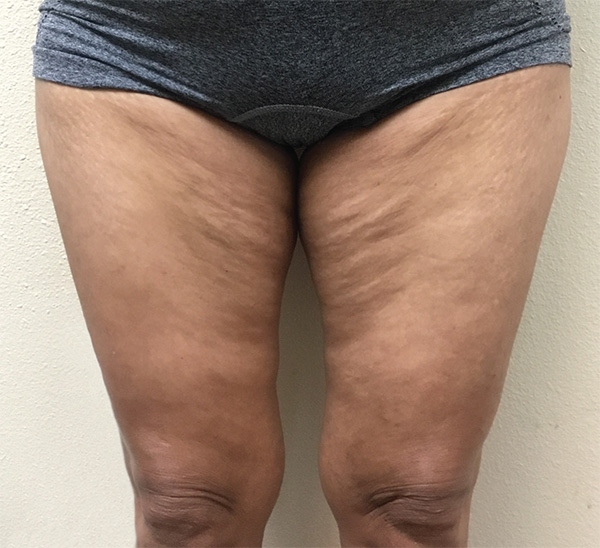
Before
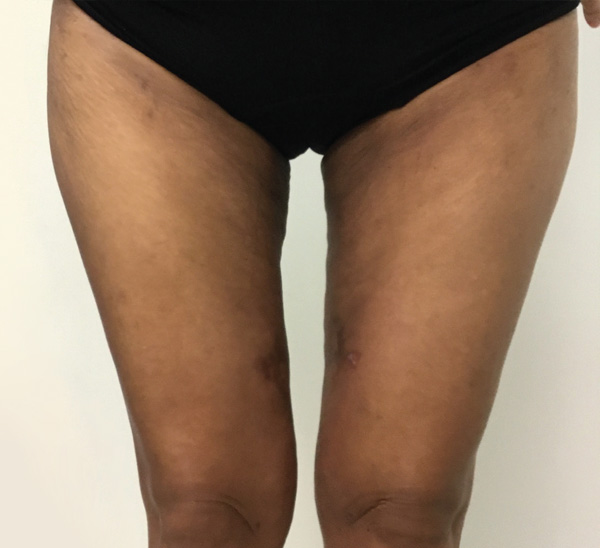
5 Weeks Post-Op
Procedure Type: Liposuction
Amount of Fat Removed: 1,100cc
Renuvion used subdermally on anterior and posterior thighs
Flanks and Inner Thighs Case Example #1
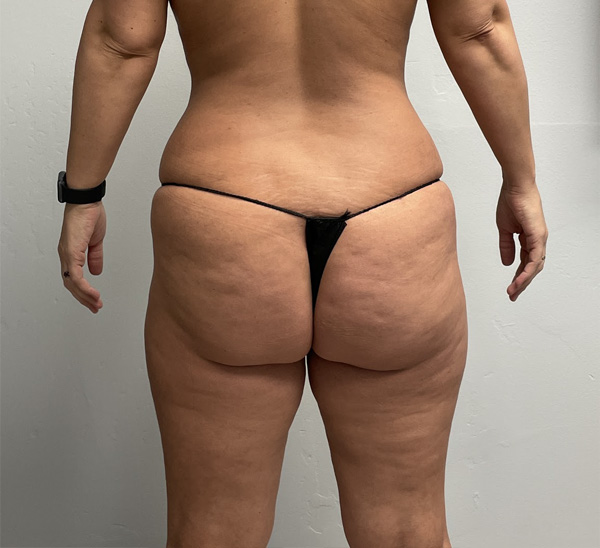
Before
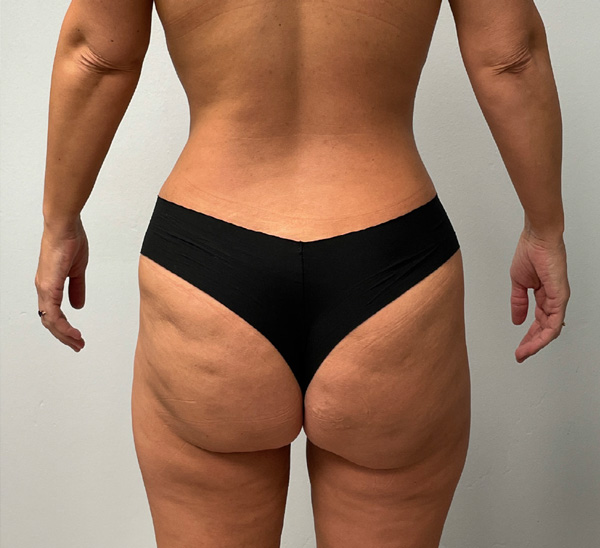
3 Months Post-Op
Procedure Type: Microaire liposuction
Amount of Fat Removed: 3000cc
Renuvion used subdermally on flanks and inner thighs
Thigh Case Example #2
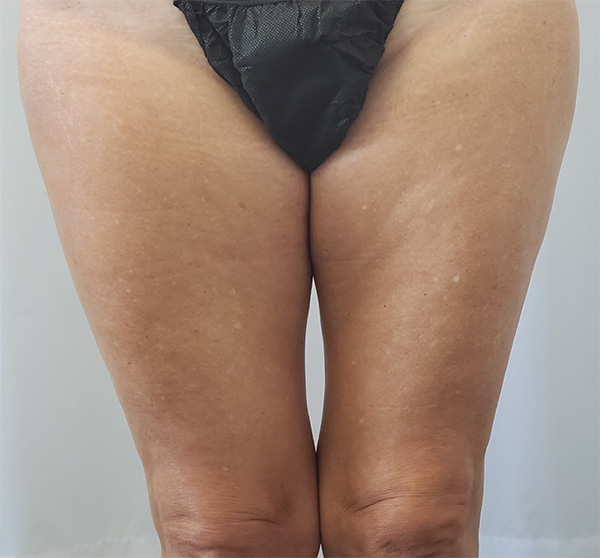
Before
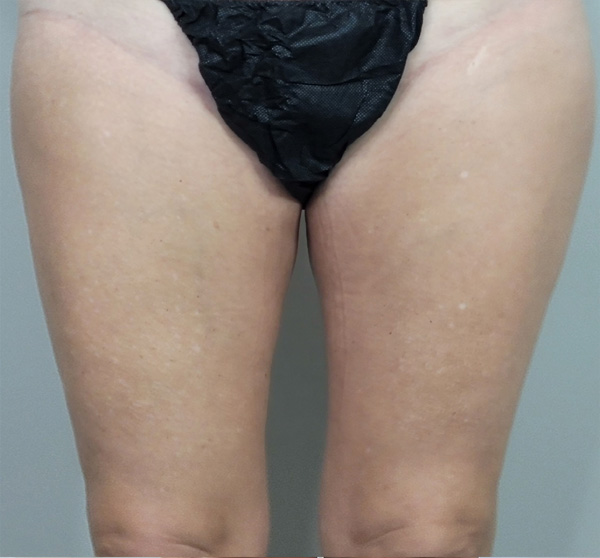
1 yr 2 mo Post-Op
Procedure Type: Suction-Assisted Liposuction
Amount of Fat Removed: 325cc
Renuvion used subdermally on medial thighs
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
References:
2. ISAPS International Survey on Aesthetic/Cosmetic Procedures 2021. https://www.isaps.org/media/vdpdanke/isaps-global-survey_2021.pdf
3. Feldman LS, et al. (eds). The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE), ISBN 978-1-4614-2073-6.
4. Chen SS, Wright NT, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: isothermal free shrinkage. Journal of Biomechanical Engineering 1997:119:372-378.
5. McDonald MB. Conductive Keratoplasty: A Radiofrequency-based Technique for the Correction of Hyperopia. Trans Am Ophthalmol Soc 2005;103:512-536.
6. Chen SS, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: pseudoelastic behavior at 37° C. J Biomech 1998;31:211-216.
8. Duncan DI and Roman S. Helium Plasma Subdermal Tissue Contraction Method of Action. Biomed J Sci & Tech Res 31(2)-2020. BJSTR. MS.ID.005075.
9. The Value of Renuvion- A Case Study with Kyle Song, MD. MM00210.00 0819 – https://www.renuvion.com/wp-content/uploads/2023/05/Value-Case-Study-Dr-Song_MM0210-00.pdf
10. The Value of Renuvion- A Case Study with Paul G. Ruff IV, MD. MM00209.00 0819 – https://www.renuvion.com/wp-content/uploads/2023/05/Value-Case-Study-Dr-Ruff_MM0209-00.pdf
11. Ruff IV, PG, Doolabh V, Zimmerman E, Gentile R. Safety and efficacy of helium plasma for subdermal coagulation. Dermatological Reviews 2020; 1-7. https://doi.org/10.1002/der2.34



